1 imágenes de Pentabromide Imágenes, fotos y vectores de stock

Phosphorus pentachloride Phosphoric acid Chemical compound Chemical
Linear Formula: PBr 5. CAS Number: 7789-69-7. Molecular Weight: 430.49. EC Number: 232-186-6. MDL number: MFCD00011437. PubChem Substance ID: 24853587. NACRES: NA.22.. Phosphorus pentabromide (PBr 5) is a bromination agent generally used to convert alcohols to bromides and in the dibromination of ketones.

How to Write the Formula for Tetraphosphorous decoxide (Phosphorous
Formula: PBr 5 Hill system formula: Br 5 P 1 CAS registry number: [7789-69-7] Formula weight: 430.494 Class: bromide Colour: orange Appearance: crystalline solid Melting point: >100°C (decomposes) Boiling point: 106°C Density: 3600 kg m -3 The following are some synonyms of phosphorus pentabromide: phosphorus pentabromide
Buy Phosphorus Pentabromide at Best Price, Phosphorus Pentabromide
The Phosphorus pentabromide molecule contains a total of 6 atom (s). There are 1 Phosphorous atom (s) and 5 Bromine atom (s). A chemical formula of Phosphorus pentabromide can therefore be written as: Br5P

Understanding Phosphorus
Molecular Formula BrP Average mass 430.494 Da Monoisotopic mass 425.565399 Da ChemSpider ID 56429 More details: Names Properties Searches Spectra Vendors Articles More Names and Synonyms Database ID (s) Validated by Experts, Validated by Users, Non-Validated, Removed by Users 232-186-6 [EINECS] 3D9WIS0BQW 7789-69-7 [RN] MFCD00011437 [MDL number]
Phosphorus pentabromide, 95, Thermo Scientific™
Chemical formula: PBr 5: Molar mass: 430.49 g mol −1: Appearance: red-yellow solid Density: 3.595 g cm −3: Melting point: 83.8 °C Boiling point: 106 °C decomp. Solubility in water: hydrolyzes Hazards;. Phosphorus pentabromide, PBr 5, is the highest bromide of phosphorus.
ChemistryPhosphorus pentabromide HandWiki
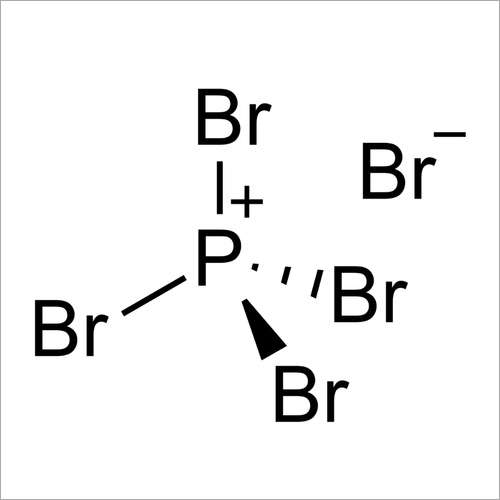
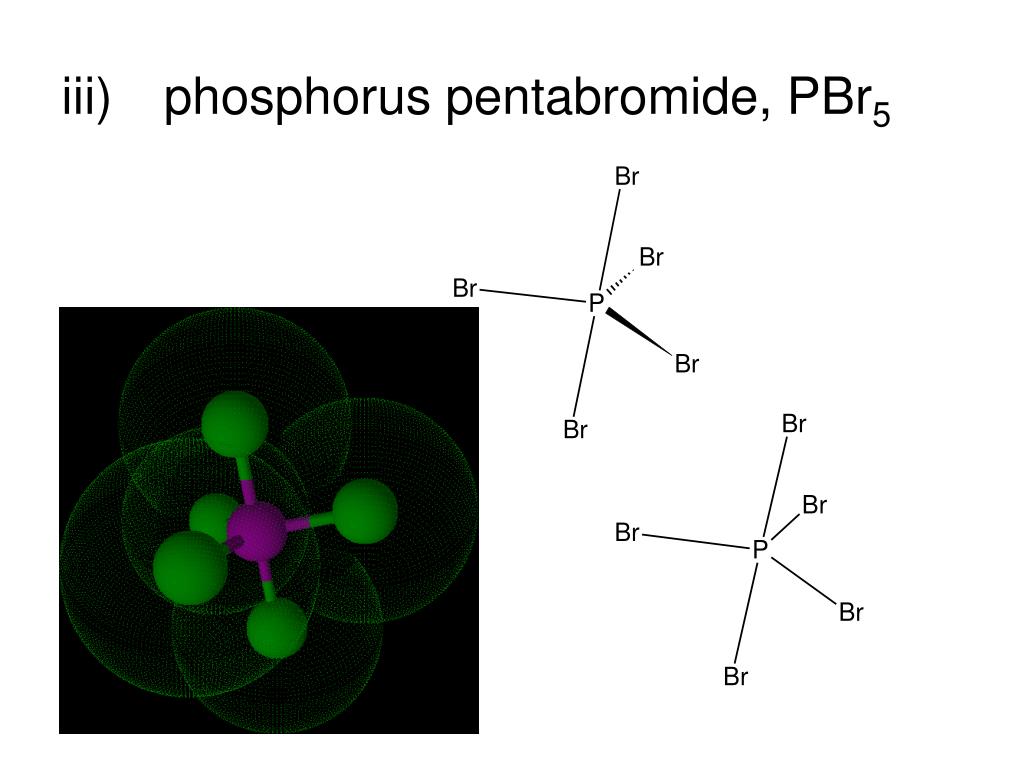
Phosphorus pentabromide is a reactive, yellow solid of formula P Br 5, which has the structure PBr 4 + Br − in the solid state but in the vapor phase is completely dissociated to PBr 3 and Br 2.Rapid cooling of this phase to 15 K leads to formation of the ionic species phosphorus heptabromide ([PBr 4] + [Br 3] −). It can be used in organic chemistry to convert carboxylic acids to acyl.

Valency of phosphorus is 3. What is the formula of its oxide, sulphide
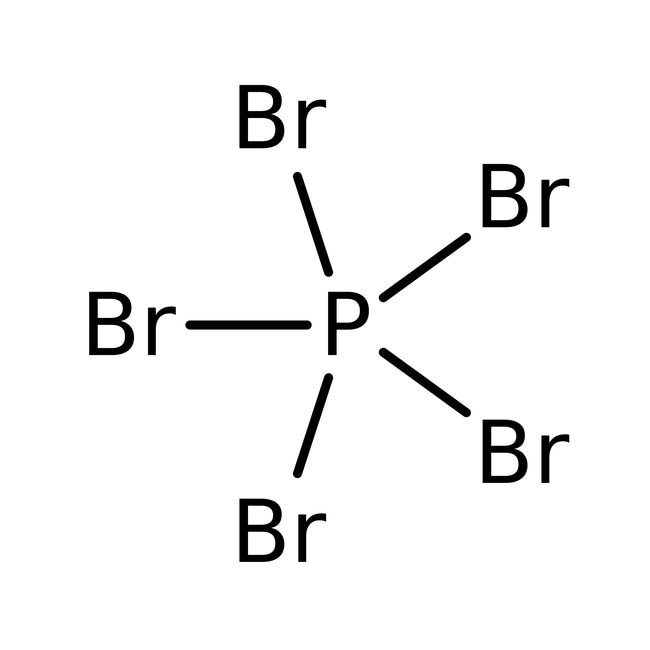
PBr5 is the chemical formula of phosphorus pentabromide, as there are phosphorous and bromine present a 1:5 proportion, as P bears the cationic part so it comes first followed by three bromide ions. PBr5 Structure 3. PBr 5 CAS number
PPT Chemical Bonding PowerPoint Presentation, free download ID6824380
Molecular Formula Br5P PBr5 Synonyms Phosphorus pentabromide 7789-69-7 Phosphorane, pentabromo- pentabromo-lambda5-phosphane phosphorus (V) bromide View More. Molecular Weight 430.49 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Dates Create: 2005-08-08 Modify: 2024-01-06 Description

How to Write the Formula for Phosphorus pentabromide YouTube
0:00 / 1:34 PBr5 Lewis Structure - How to Draw the Lewis Structure for PBr5 Wayne Breslyn 724K subscribers Join Subscribe Subscribed 123 30K views 10 years ago A step-by-step explanation of how.

Products
Linear Formula: PBr5 CAS Number: 7789-69-7 Molecular Weight: 430.49 EC Number: 232-186-6 MDL number: MFCD00011437 PubChem Substance ID: 24853587 NACRES: NA.22 Pricing and availability is not currently available. Recommended Products Sigma-Aldrich 376949 Phosphorus (V) oxybromide View Price and Availability Sigma-Aldrich 256536 Phosphorus tribromide

Phosphorus pentabromide PBr5. Chemical reactions YouTube
Phosphorus pentabromide written as PBr5 in the chemistry equations is a reactive yellow solid. The compound has one molecule of Phosphorus and five Bromine molecules. Bromine is a halogen from Group 17 of the periodic table. Halogens are highly reactive and electronegative molecules.
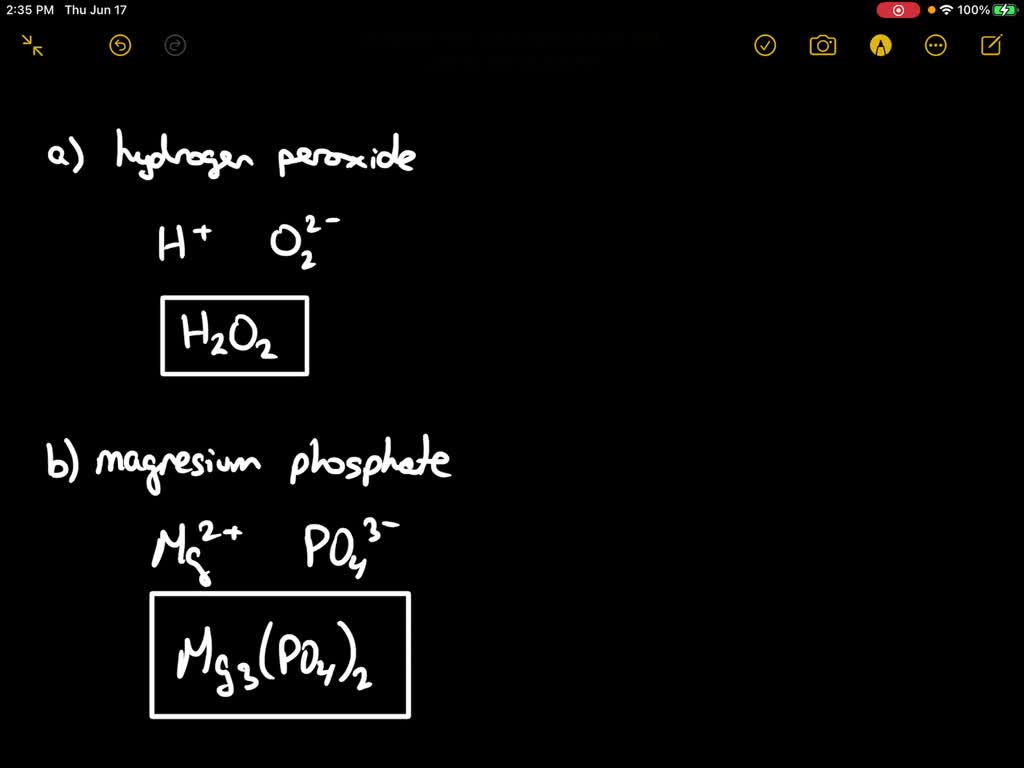
SOLVED(4 points) Write the formula for the following compounds
Phosphorus pentabromide is a reactive, unstable, yellow solid chemical compound with the formula PBr5. This compound has the structure PBr 4+ Br − in the solid state, but in the vapor phase is completely dissociated to PBr 3 and Br 2 . Contents [ hide ] 1 Properties 1.1 Chemical 1.2 Physical 2 Availability 3 Preparation 4 Projects 5 Handling

H3po4
Phosphorus pentabromide is a reactive, yellow solid of formula PBr5, which has the structure +Br− in the solid state but in the vapor phase is completely dissociated to PBr3 and Br2. Rapid cooling of this phase to 15 K leads to formation of the ionic species phosphorus heptabromide .

Phosphorus Pentabromide SA.pdf DocDroid
0:00 / 1:30 How to Write the Formula for Phosphorus pentabromide Wayne Breslyn 718K subscribers 3.4K views 2 years ago In this video we'll write the correct formula for Phosphorus pentabromide.

Phosphorus Pentabromide SA.pdf DocDroid
Formula: Br 5 P Molecular weight: 430.494 CAS Registry Number: 7789-69-7 Information on this page: Data at other public NIST sites: X-ray Photoelectron Spectroscopy Database, version 4.1 Options: Switch to calorie-based units Go To: Data from NIST Standard Reference Database 69: NIST Chemistry WebBook

1 imágenes de Pentabromide Imágenes, fotos y vectores de stock
PBr5 or phosphorus pentabromide is a compound of phosphorus and bromine which is yellow colored (solid) in appearance. It has a got huge application in organic chemistry. So we will study the bonding in PBr5 by understanding the PBr5 lewis structure. Some facts about phosphorus pentabromide This compound has a molar mass of around 430.49 g/mol.